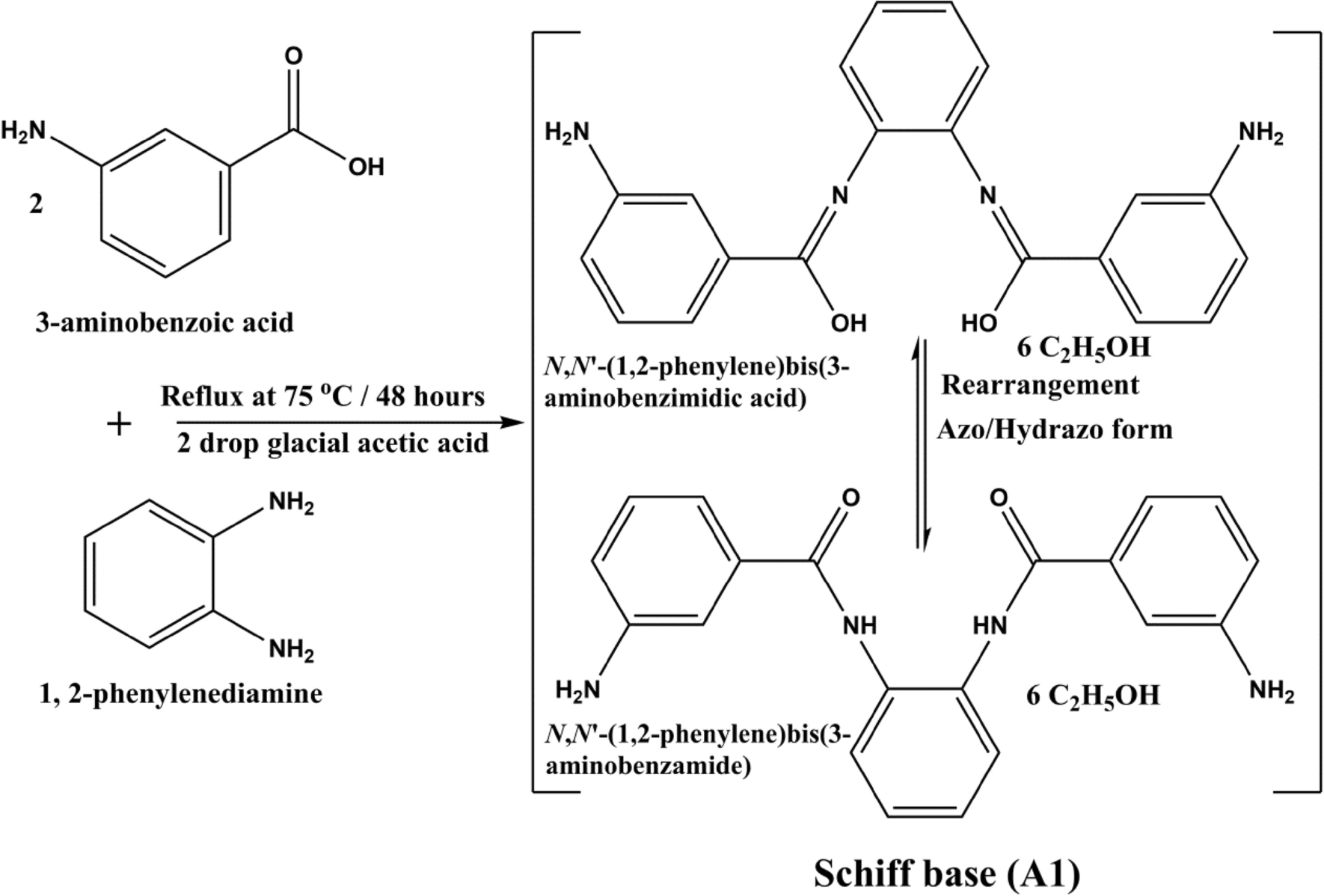
Molecules | Free Full-Text | Transesterification of Lactic Acid Oligomers with Ethanol, a Way to Anhydrous Ethyl Lactate: A Kinetic Study

Growth Pattern, Stability, and Properties of Complexes of C2H5OH and nCO2 (n = 1–5) Molecules: A Theoretical Study | ACS Omega
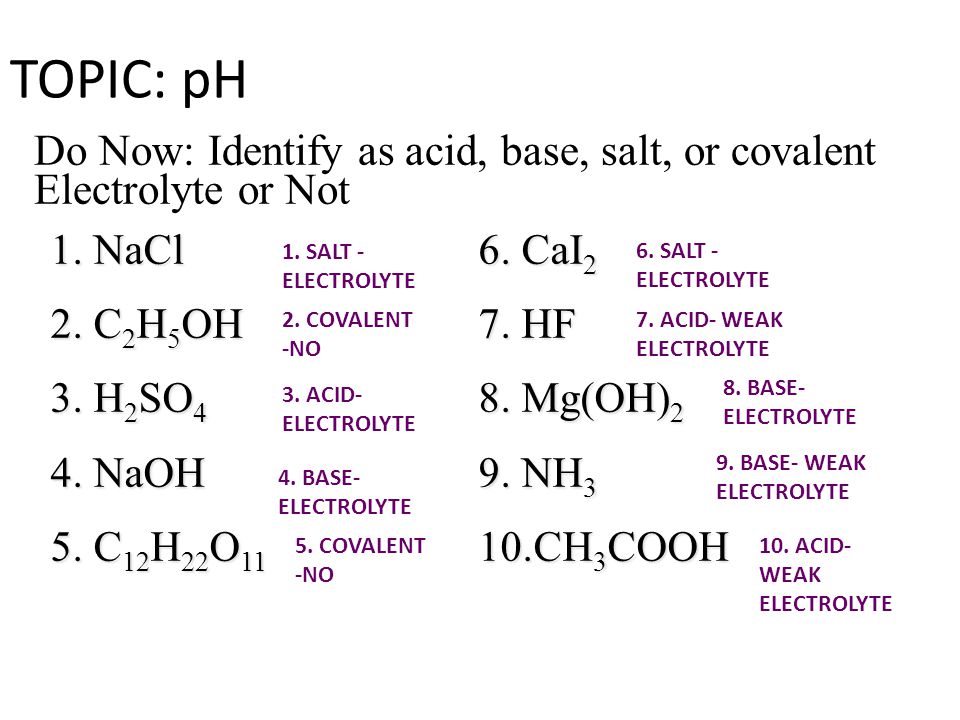
TOPIC: pH Do Now: Identify as acid, base, salt, or covalent Electrolyte or Not 1. NaCl 6. CaI2 2. C2H5OH 7. HF 3. H2SO4 8. Mg(OH)2 4. NaOH 9. NH3 5. C12H22O ppt download
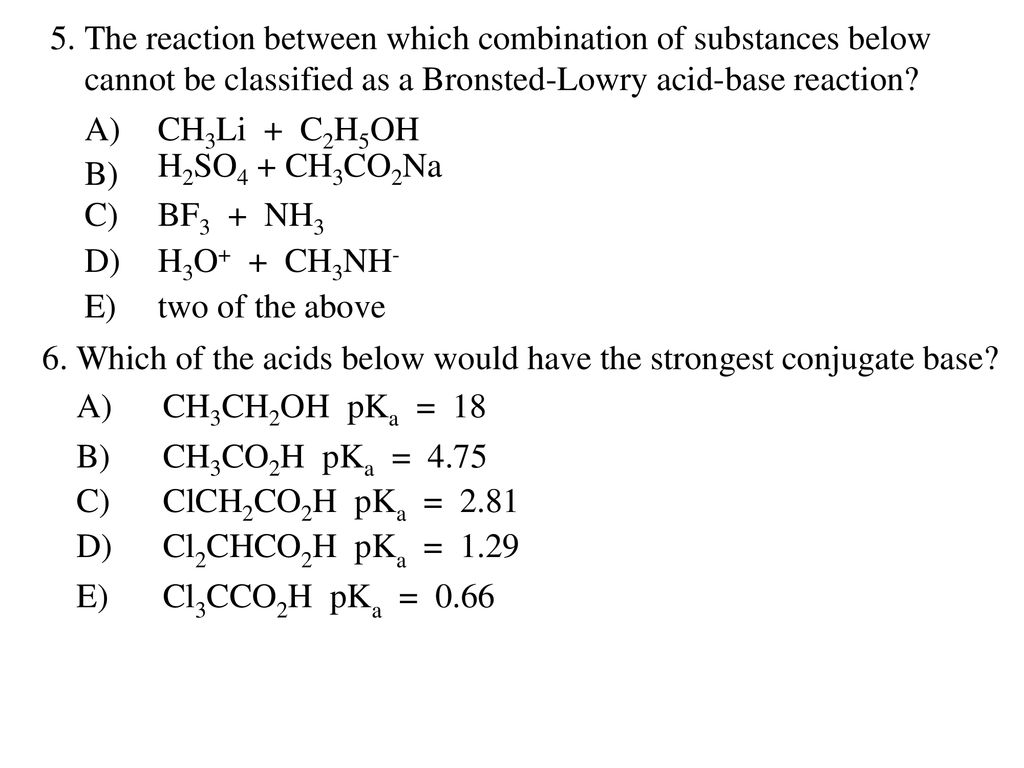
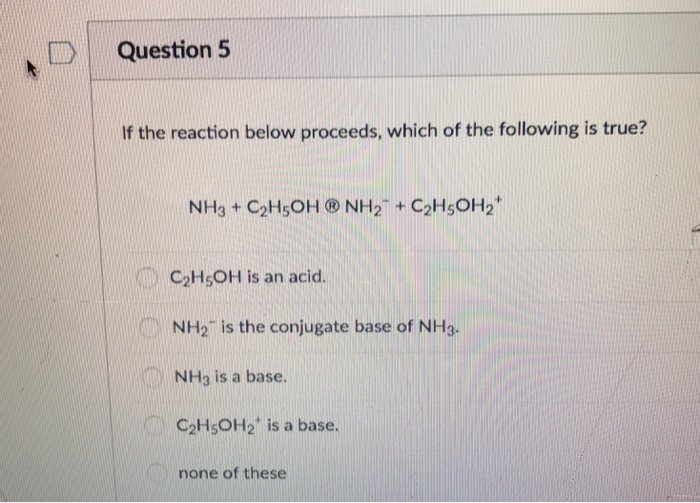
1. Which of the following is not both a Bronsted-Lowry acid and a Bronsted-Lowry base? A) HSO4- (hydrogen sulfate) B) H2PO4- (dihydrogen phosphate) - ppt download

Reagents and conditions: (a) CCl3CH(OH)2, NH2OH. HCl, 45min heating;... | Download Scientific Diagram

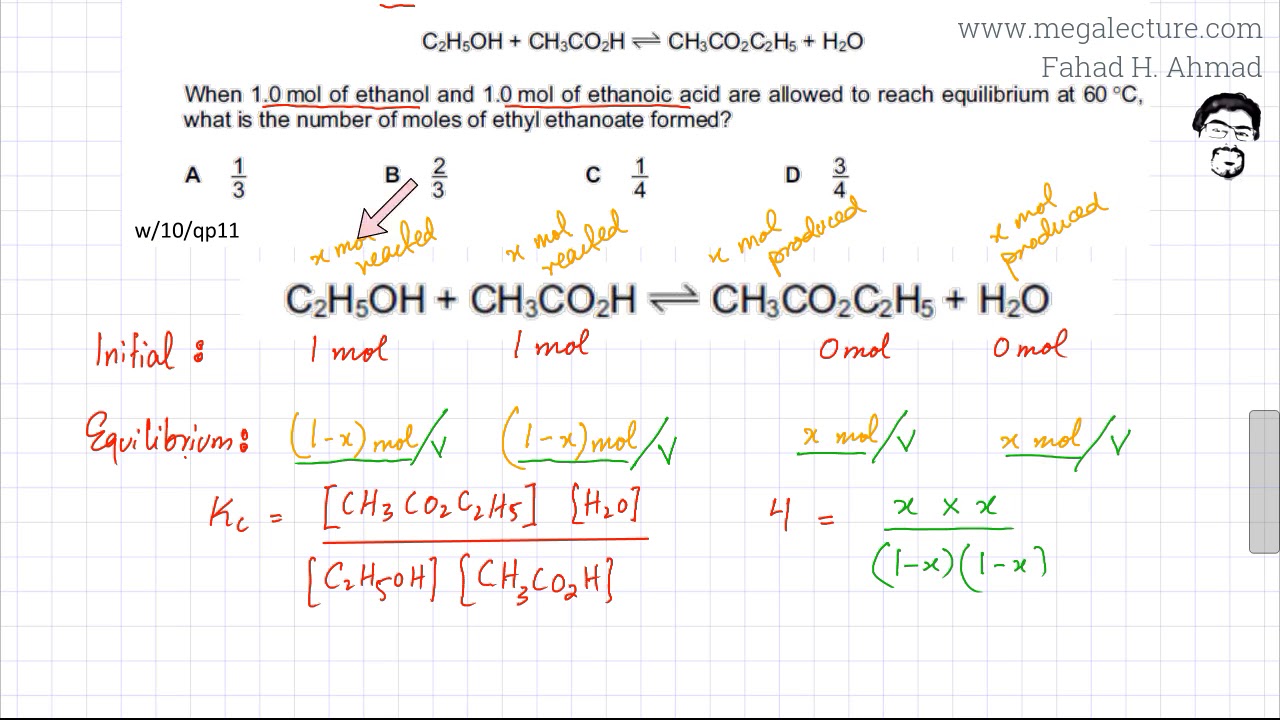
In an experiment starting with 1 mol C(2)H(5)OH, 1 mol CH(3)COOH, and 1 mol of water, the equilibrium mixture mixture of analysis showa that 54.3% of the acid is eaterified. Calculate K(c).