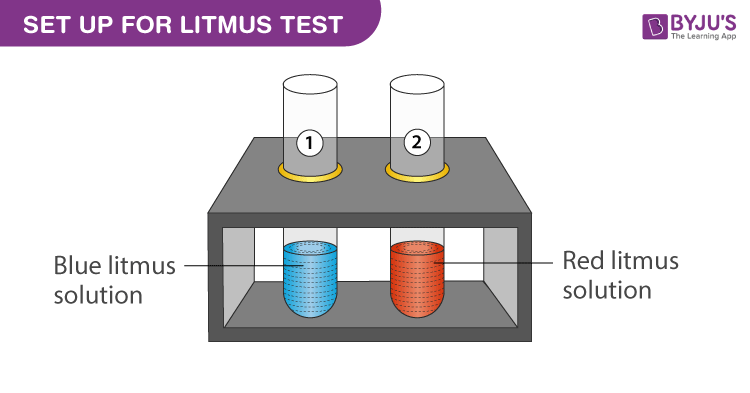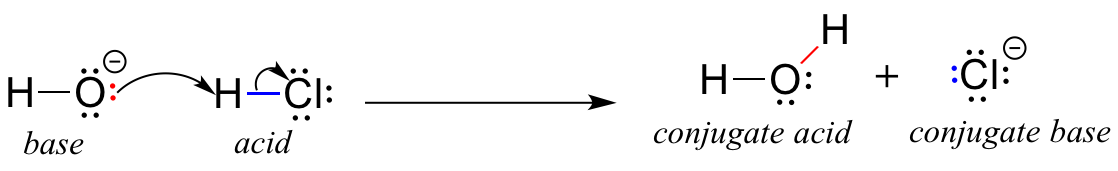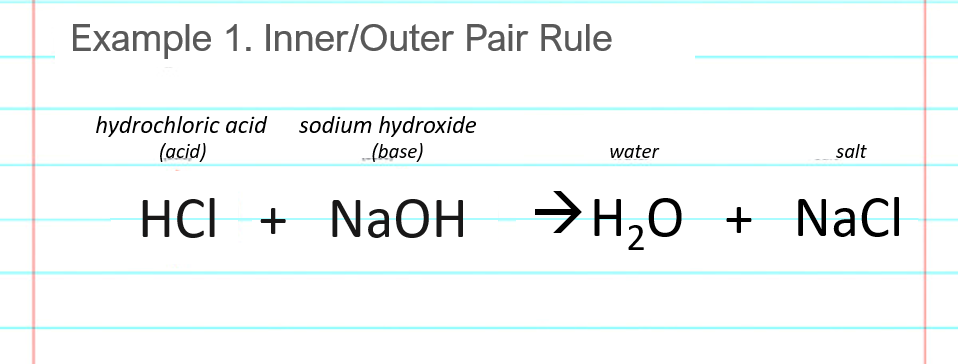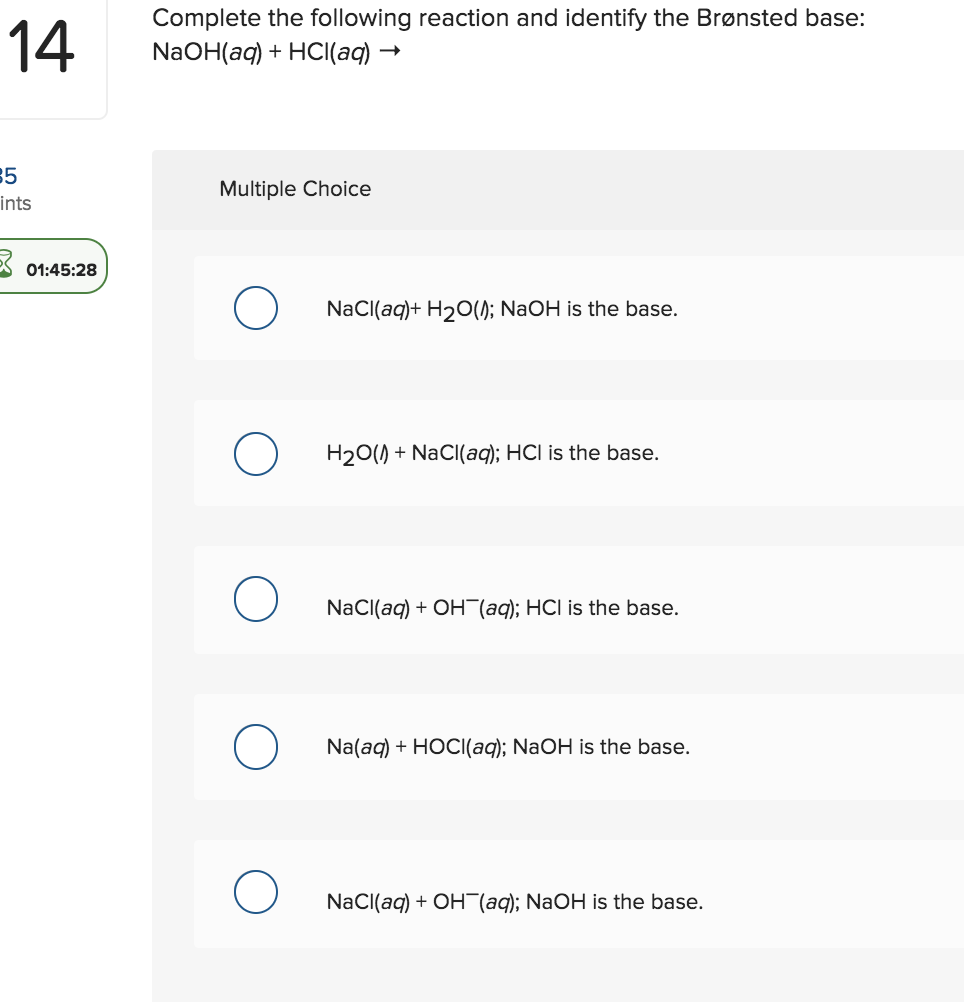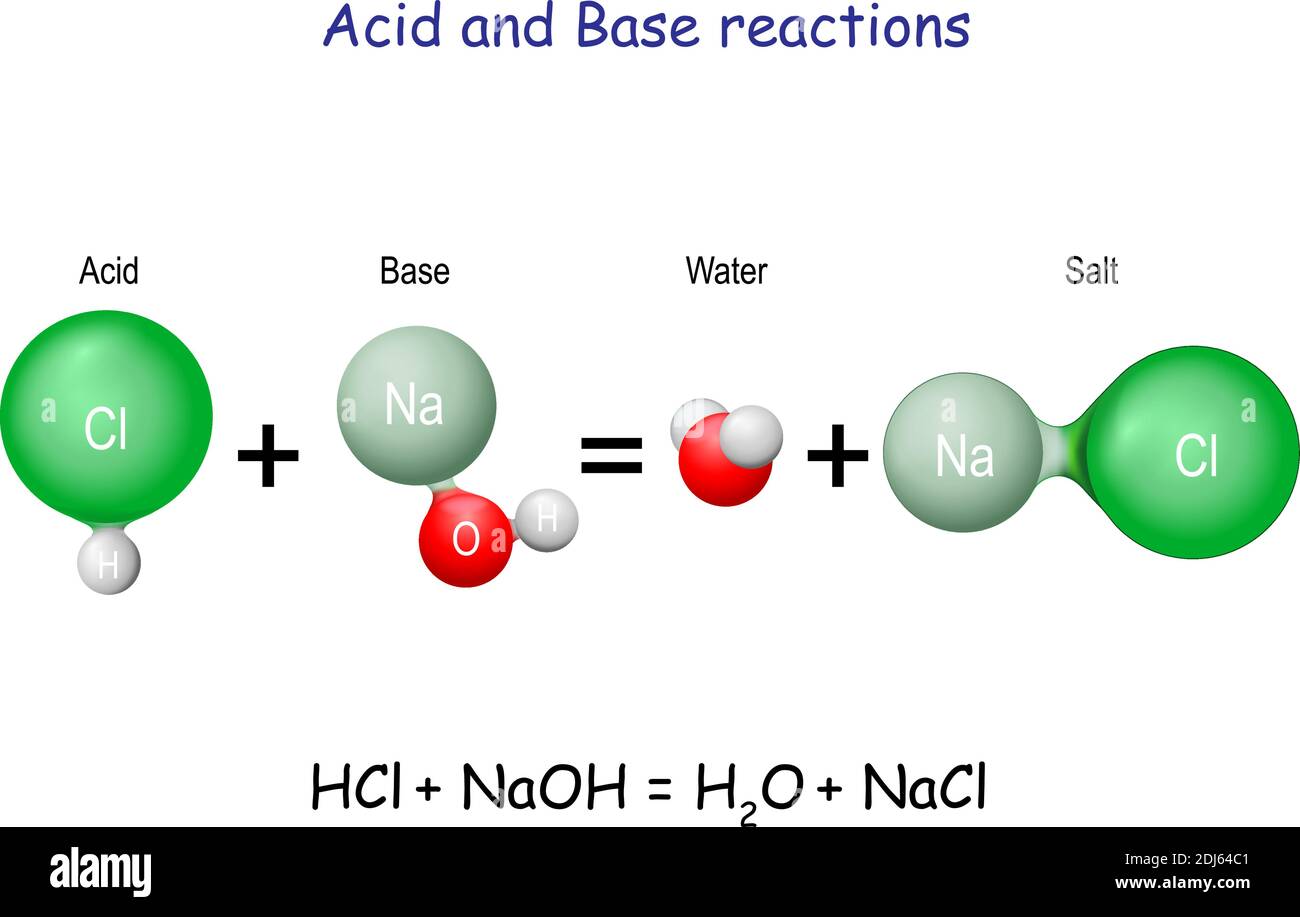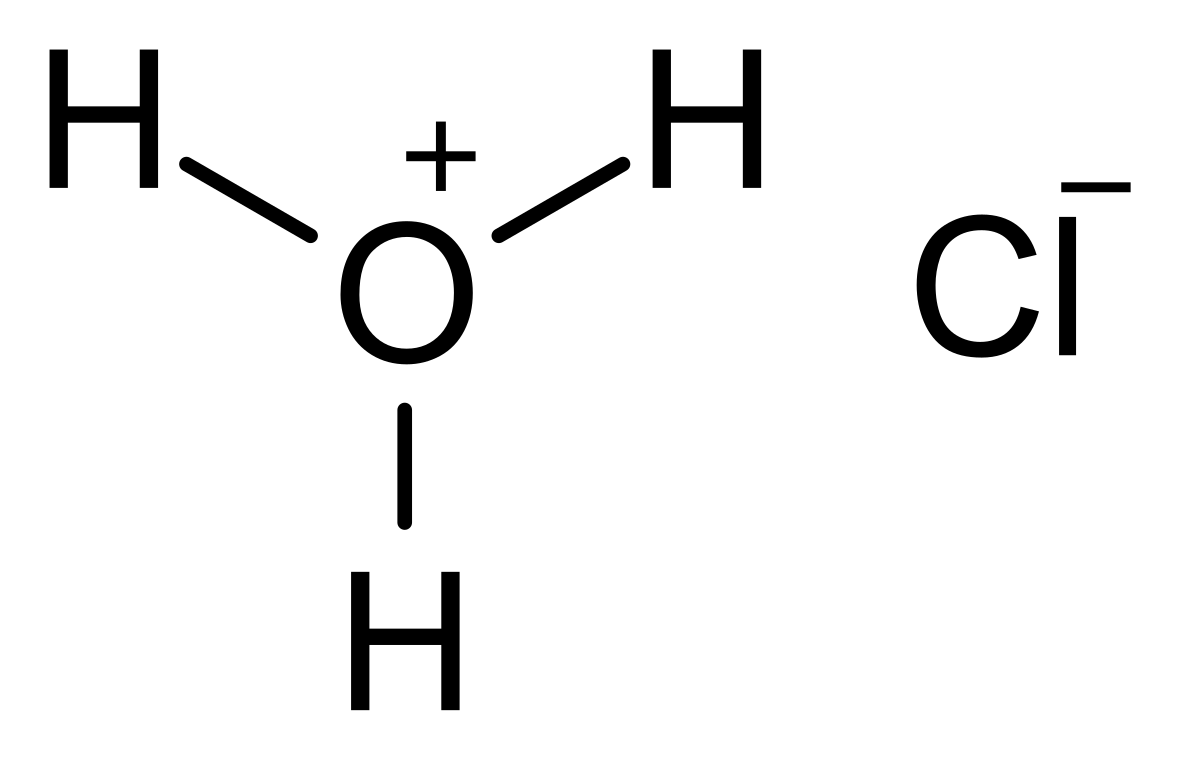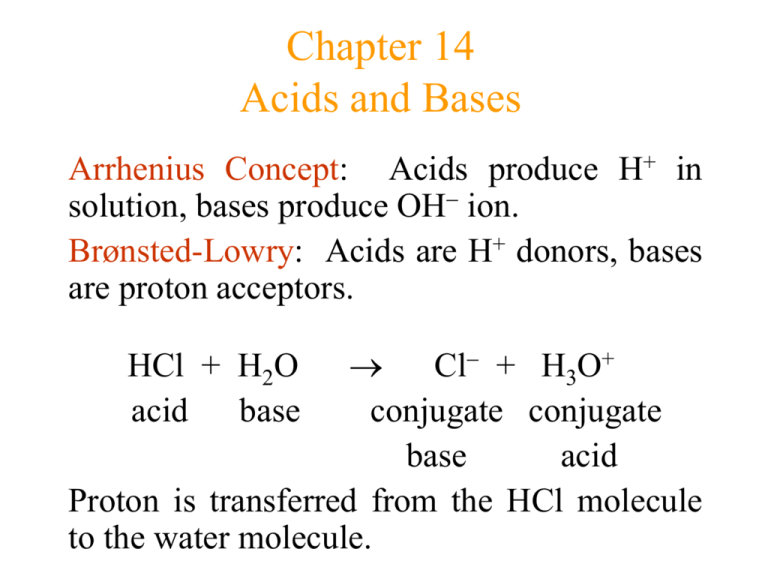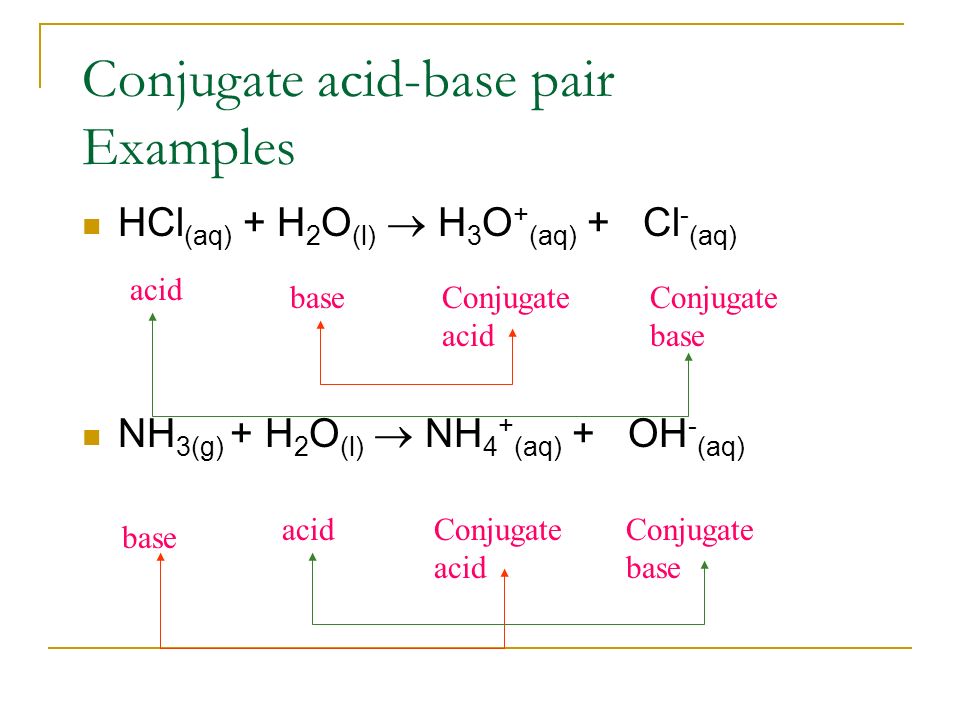
pH and Buffers Acids and Bases Acids: H + donors HCl H + + Cl - CH 3 COOH CH 3 COO - + H + Bases: H + acceptors NaOH + H + Na + + H 2 O - ppt download
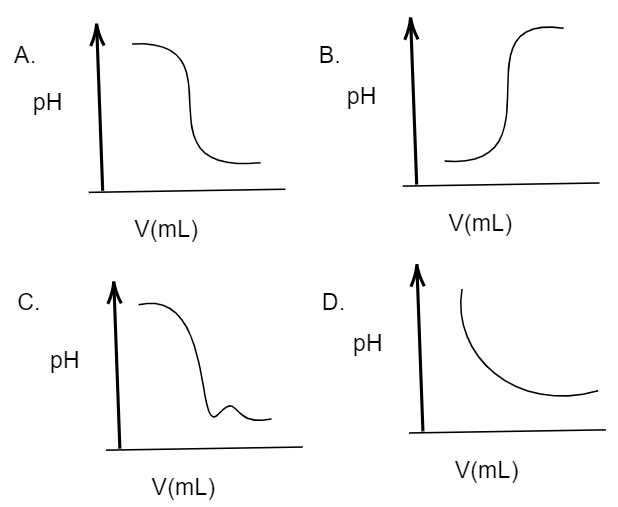
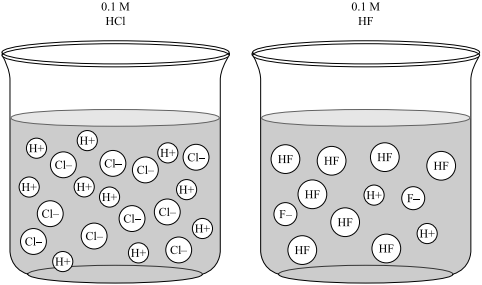
In an acid-base titration, $0.1M$ $HCl$ solution was added to the $NaOH$ solution of unknown strength. Which of the following correctly shows the change of pH of the titration mixture in this

Write a chemical equation for the acid-base reaction that occurs when p-phenetidine is dissolved in HCl. Why is HCl used instead of just plain DI water? | Homework.Study.com