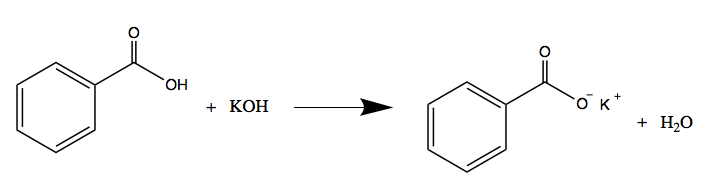
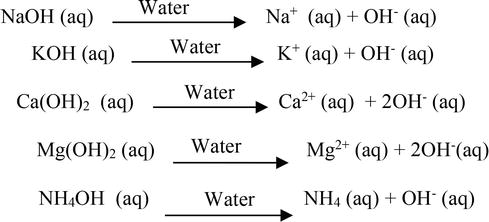
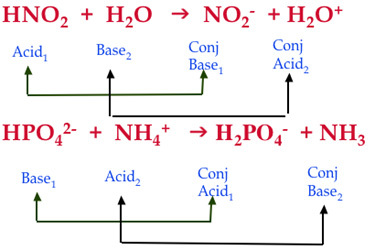
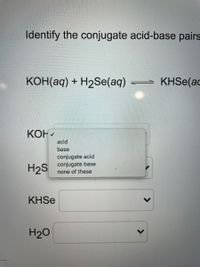
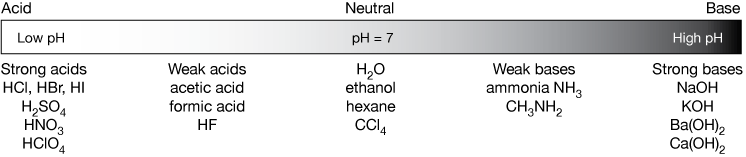
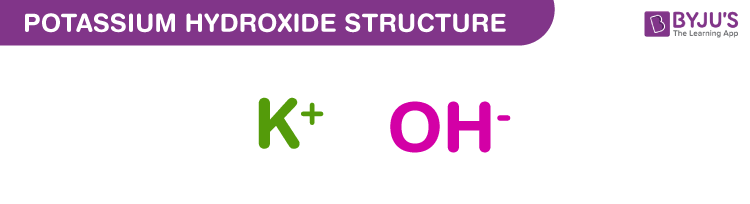
Write the neutralization reaction for the following acid and base: HCl_{(aq)} and KOH_{(aq)}. | Homework.Study.com

Solubility Data for the KOH–K2CO3–K3VO4–H2O System at (313.15 and 353.15) K | Journal of Chemical & Engineering Data
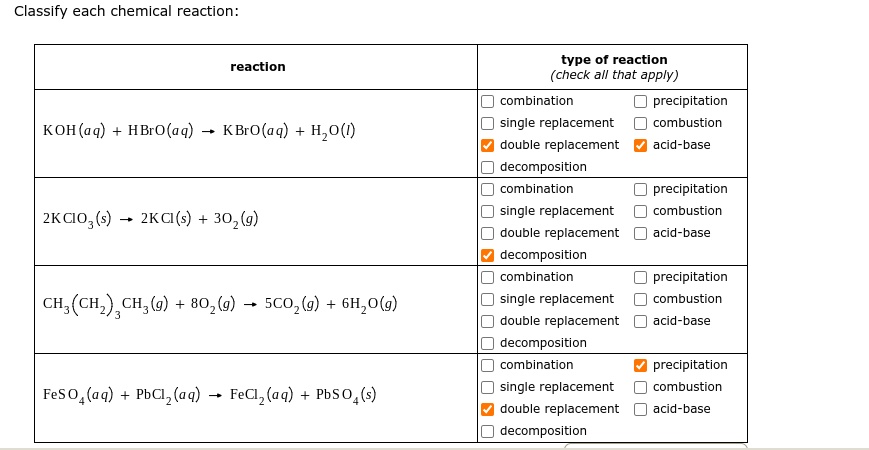
SOLVED: Classify each chemical reaction: type of reaction (check all that apply) combination precipitation reaction KOH(aq) + HBrO(aq) KBrO(aq) Hzo() single replacement double replacement combustion acid-base decomposition combination precipitation ...