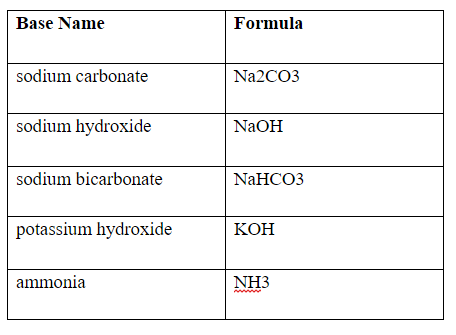
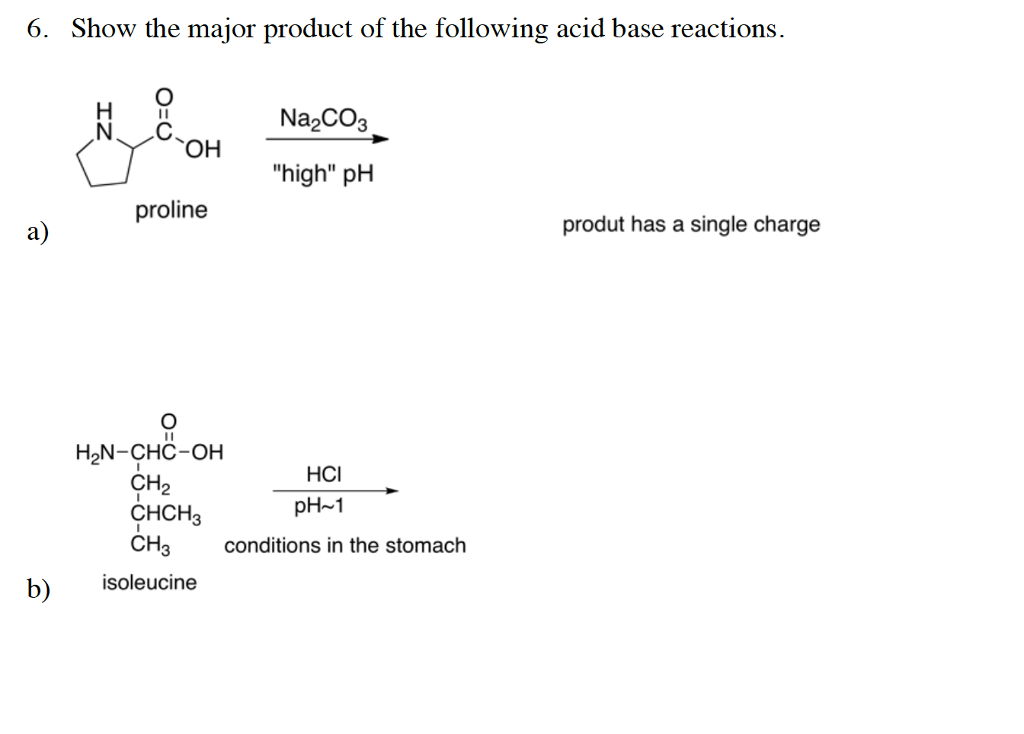
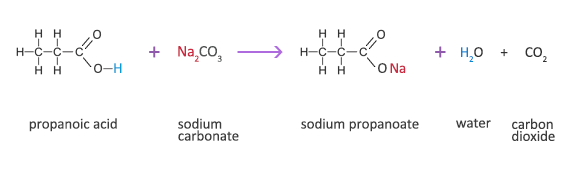
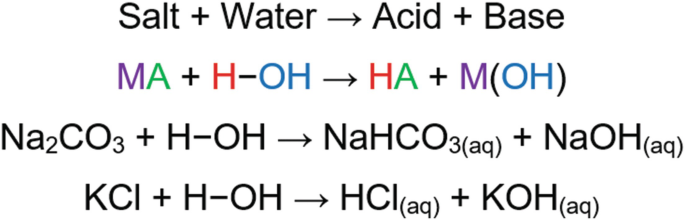
Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange
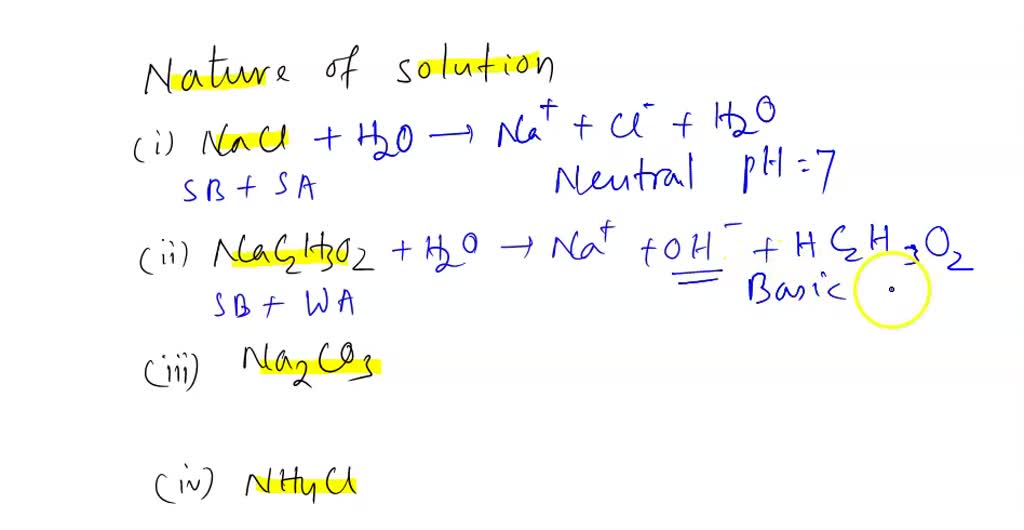
SOLVED: For the following compounds, predict whether the solution is acidic, basic or neutral and why: (i) NaCl, (ii) NaC2H3O2, (iii) Na2CO3, (iv) NH4Cl.

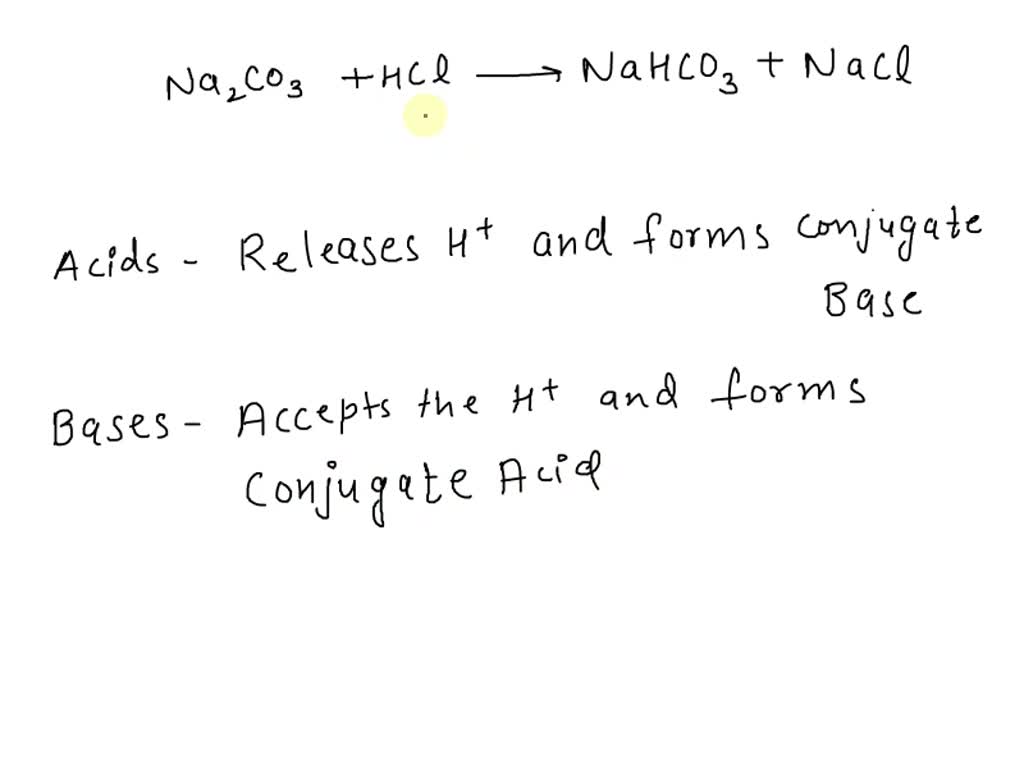
Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid | Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid Hello, Chemistry Enthusiasts! For today's