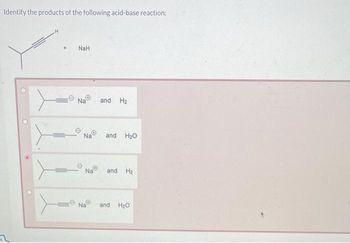
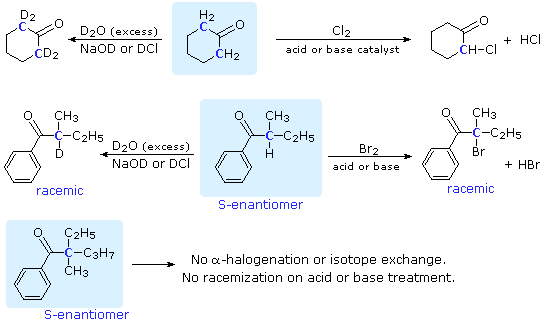
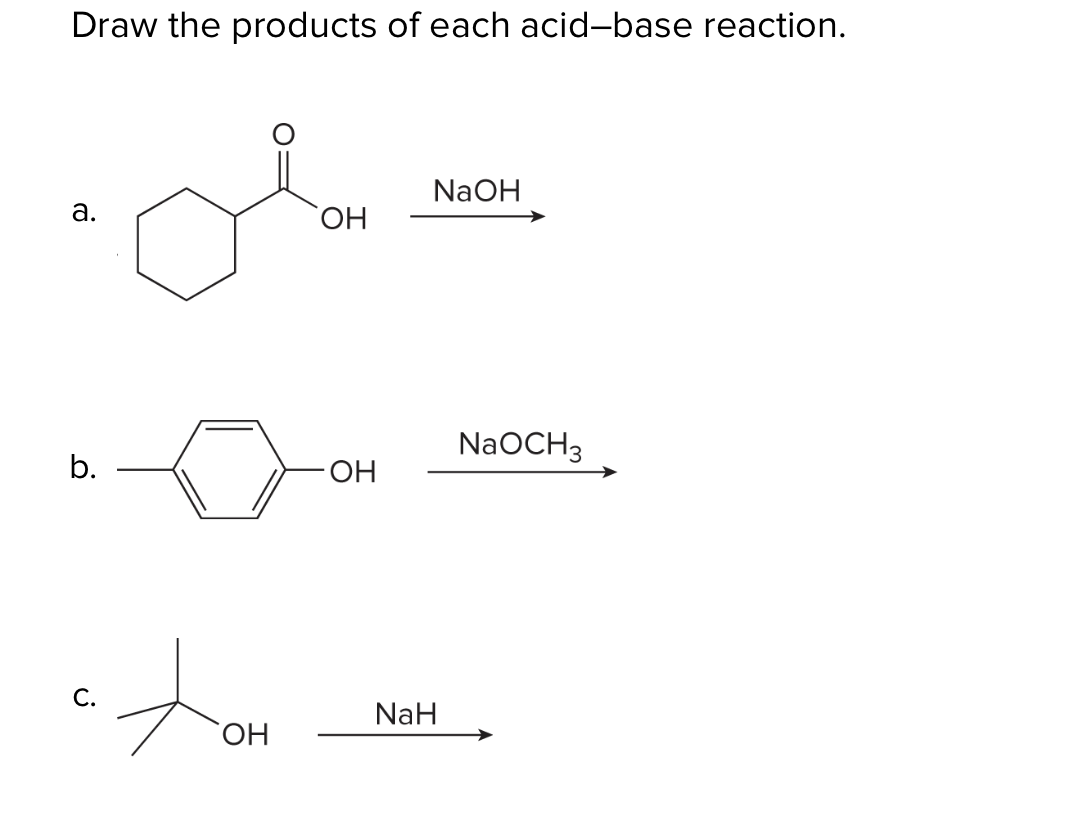
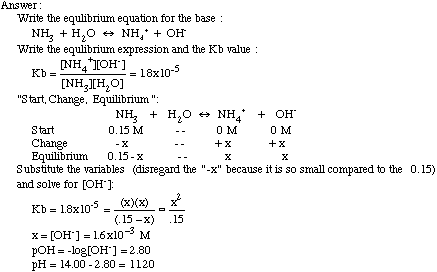SOLVED: Sodium hydride (NaH) is convenient source Of the hydride anion (H ) the conjugate base of Hz: In contrast t0 other hydride reagents that we will learn about next semester; NaH

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...
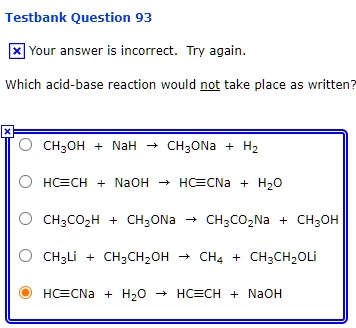
SOLVED: Testbank Question 93 Your answer incorrect Try again Which acid base reaction would not take place as written CHzOH Nah CHzONa HCCH NaOH HCCNa Hzo CH;COzH CHzONa CH;COzNa CHzOH CHzLi CHzCHzOH

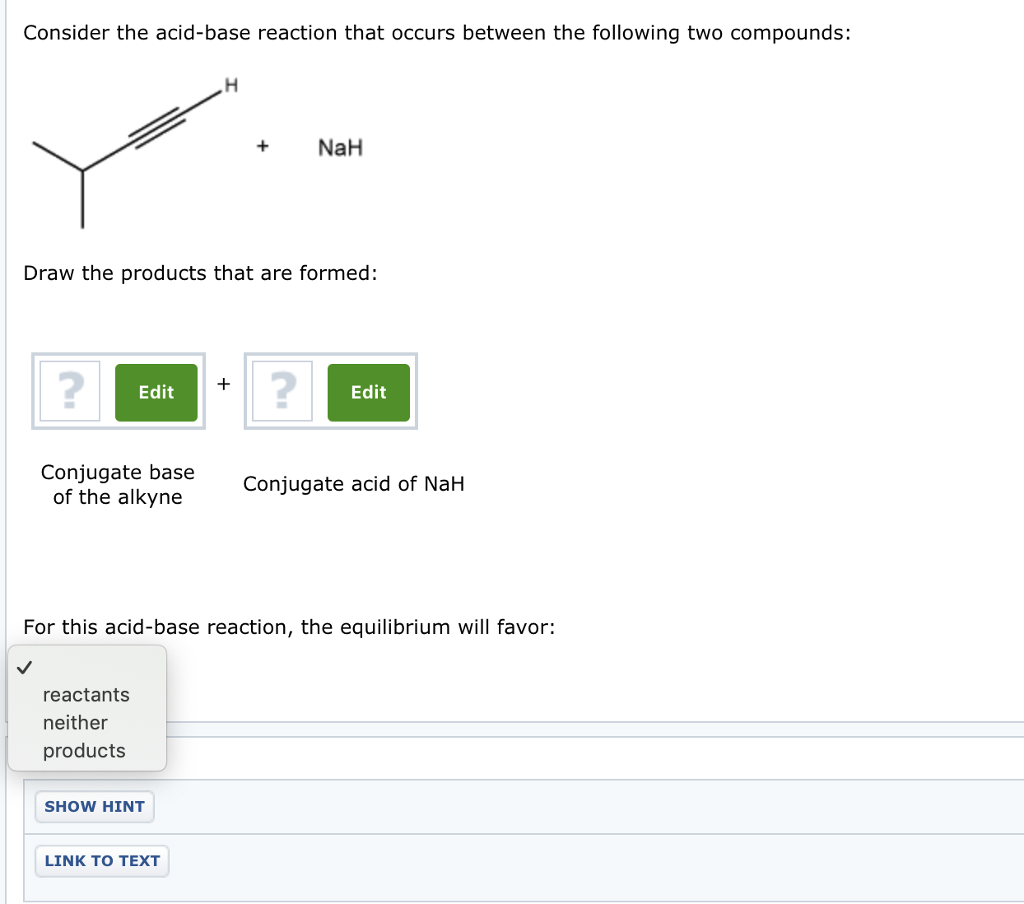
Which of the following acid-base reactions will occur? f. H-C-=CNa+CH3OH g. H-C-=CH+CH3Li h. H-C-=CH+NaH i. H-C-=CH+NaCN j. H-C-=CNa+CH3COOH

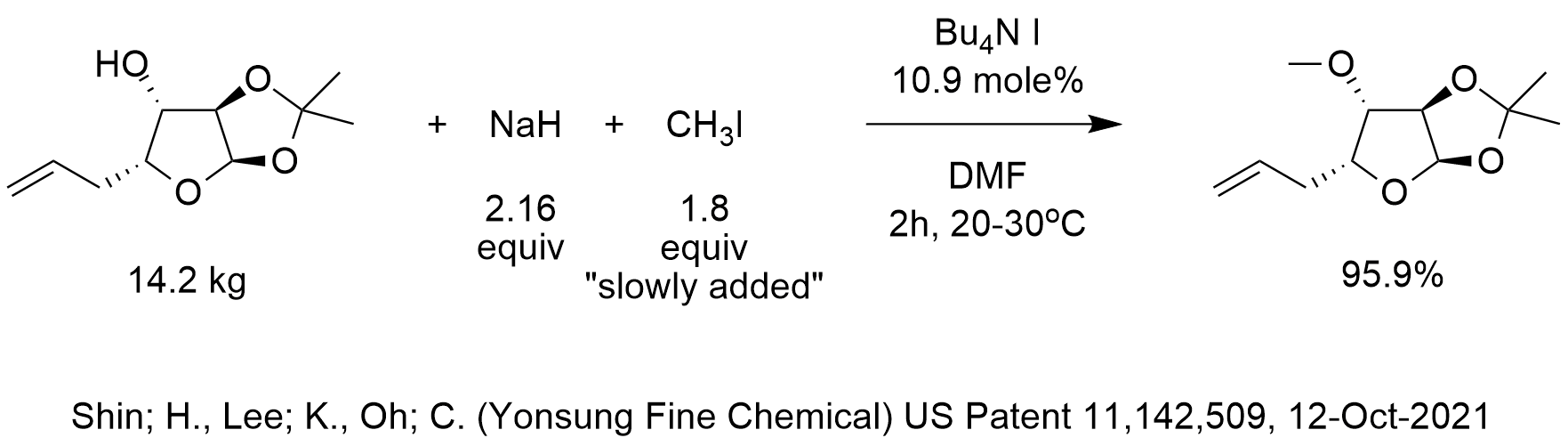
organic chemistry - Can NaH open the epoxide ring to form alcohol? If so, how? - Chemistry Stack Exchange

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

OneClass: The pKa of the conjugate acid of sodium hydride (NaH) is about 35, the pKa of sodium hydrox...

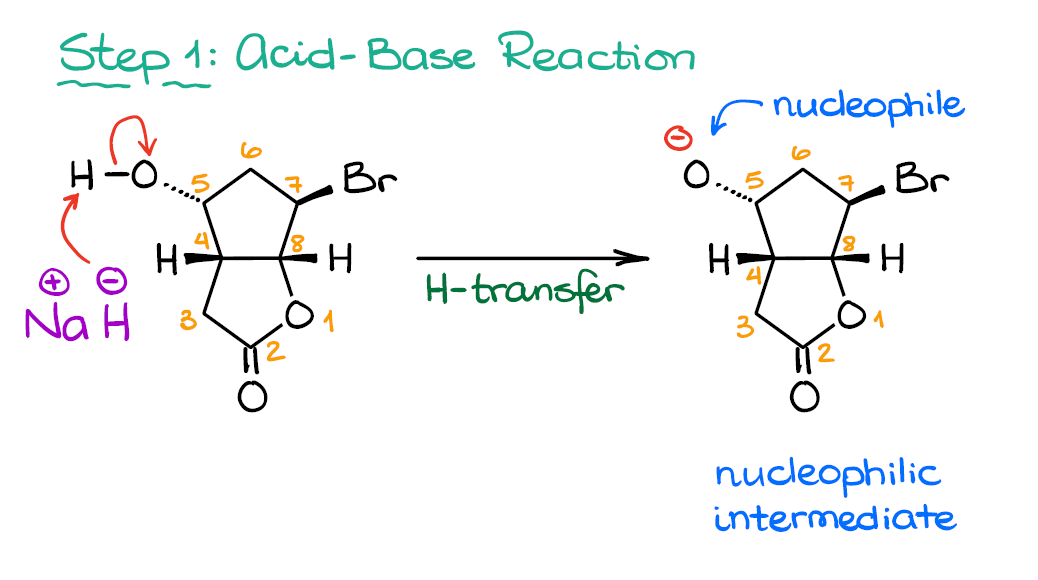
What product is formed when the following compound is treated with NaH? The following acid-base reactions were a step in a synthesis of a commercially available drug. | Homework.Study.com