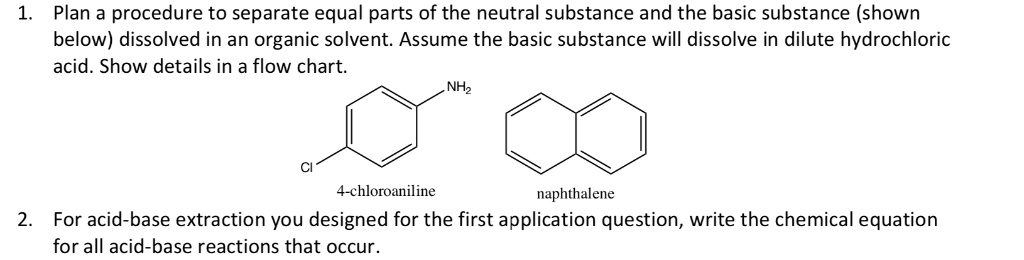
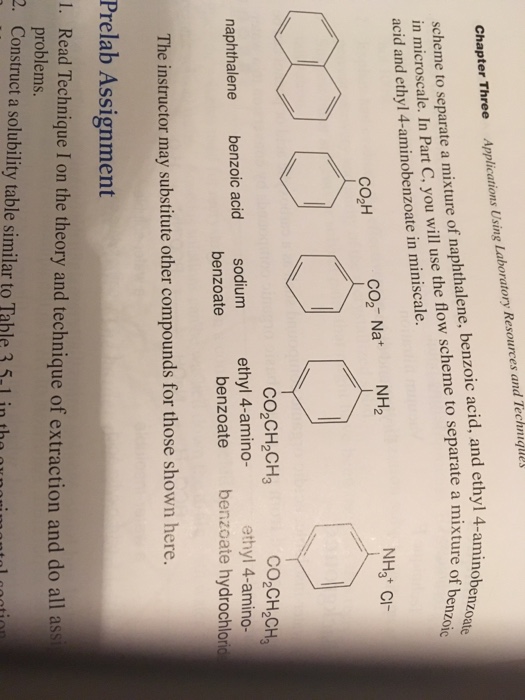
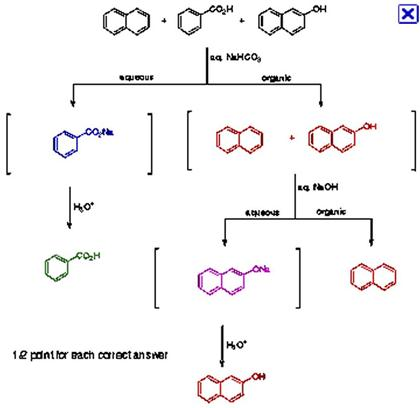
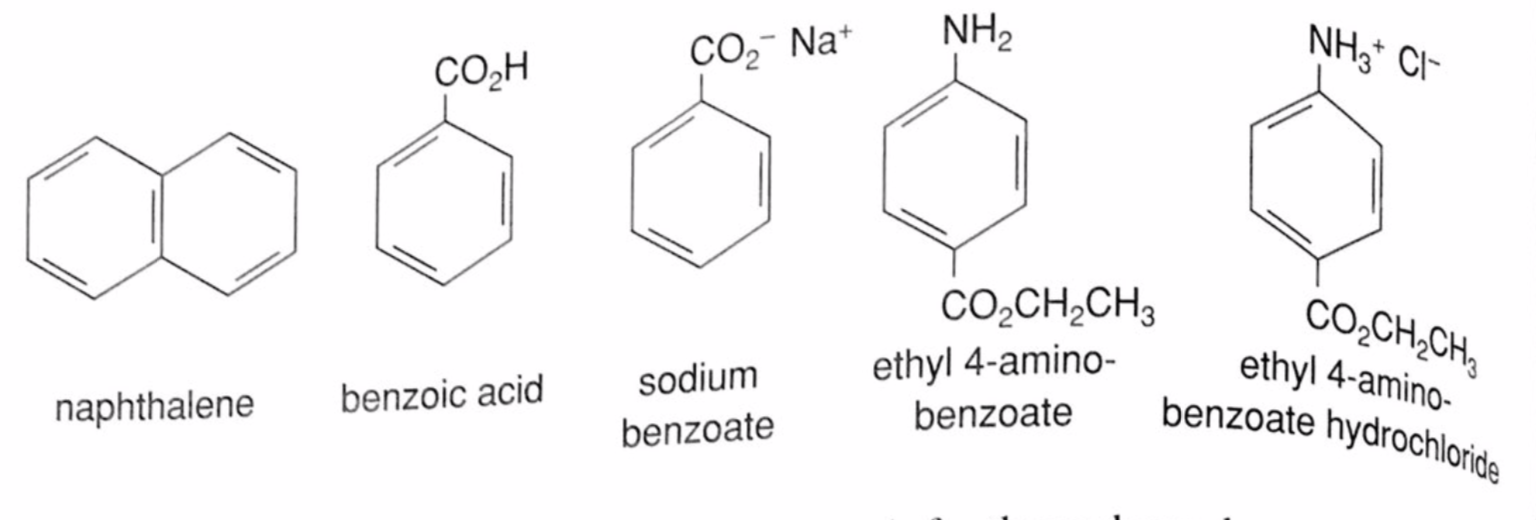
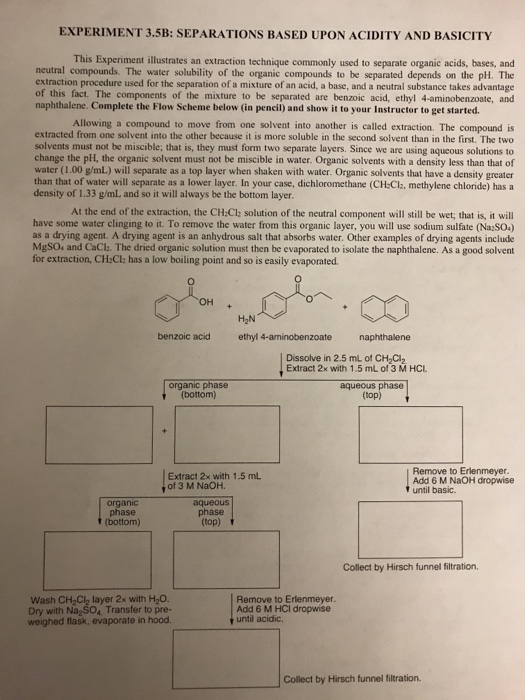
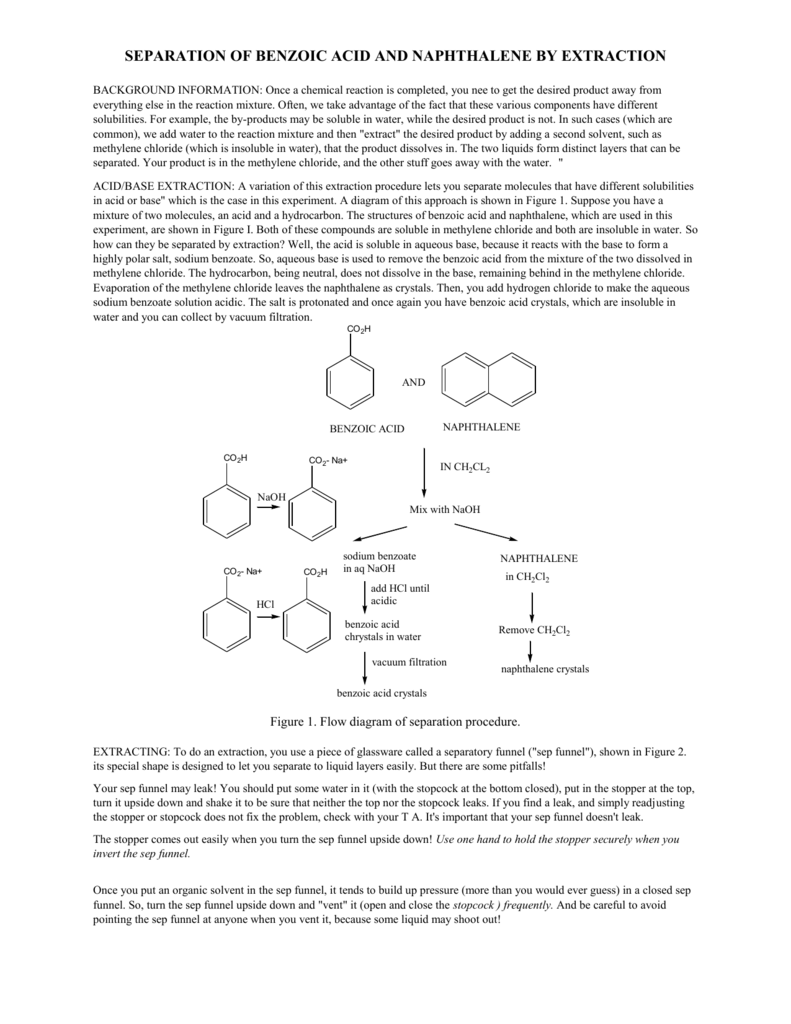
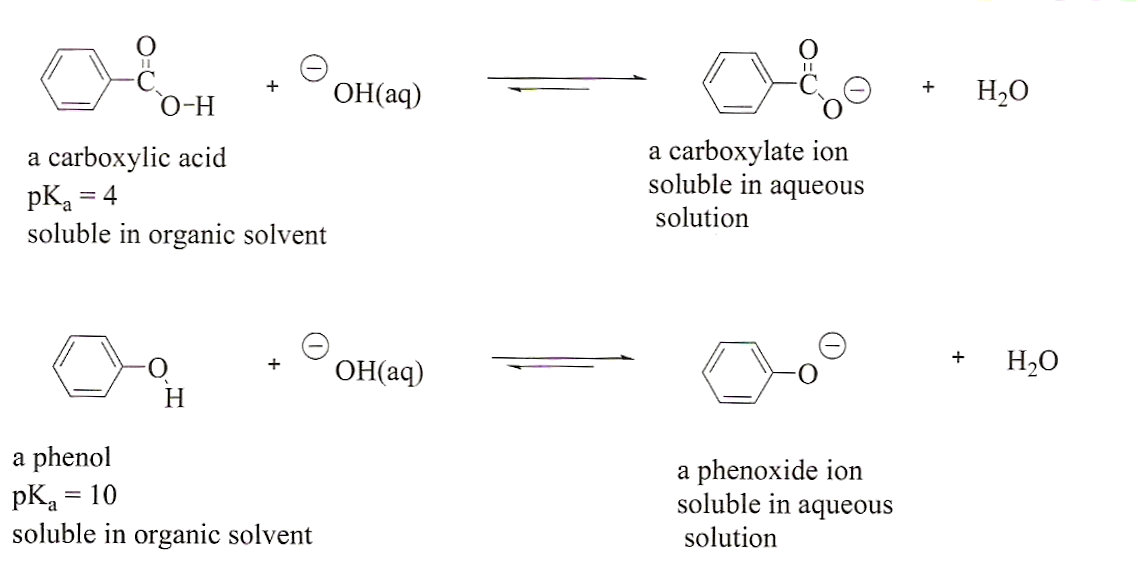
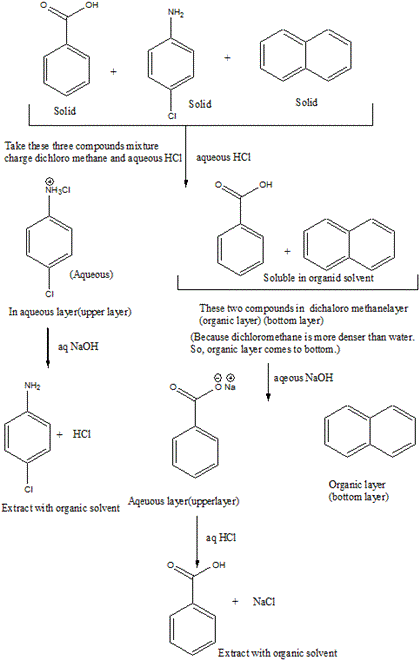
A mixture contains benzoic acid, 4-chloroaniline, and naphthalene. The 4-chloroaniline is separated first by extraction with hydrochloric acid. Since no phenolic compound is present in this mixture, t | Homework.Study.com

Write out a detailed flow scheme for the separation of naphthalene, benzoic acid and aniline. Use H_2O, HCl, NaOH and methylene chloride reagent/solvents for this. Note that benzoic acid and aniline forms
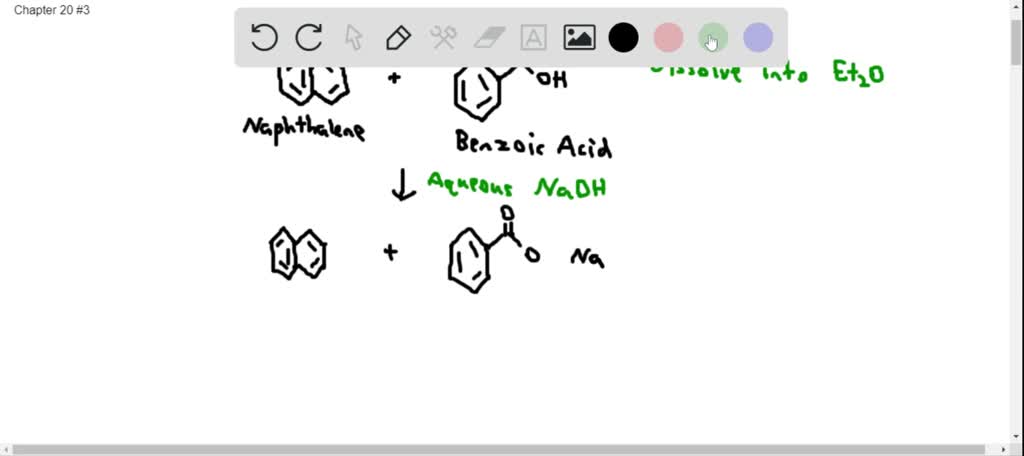
SOLVED:Assume you have a mixture of naphthalene and benzoic acid that you want to separate. How might you take advantage of the acidity of one component in the mixture to effect a

SOLVED: HW4 A student wishes t0 separate mixture of the two compounds shown below They intend t0 use liquid-liquid extraction t0 do so Aniline Naphthalene Aniline has an amine fiunctionality and naphthalene