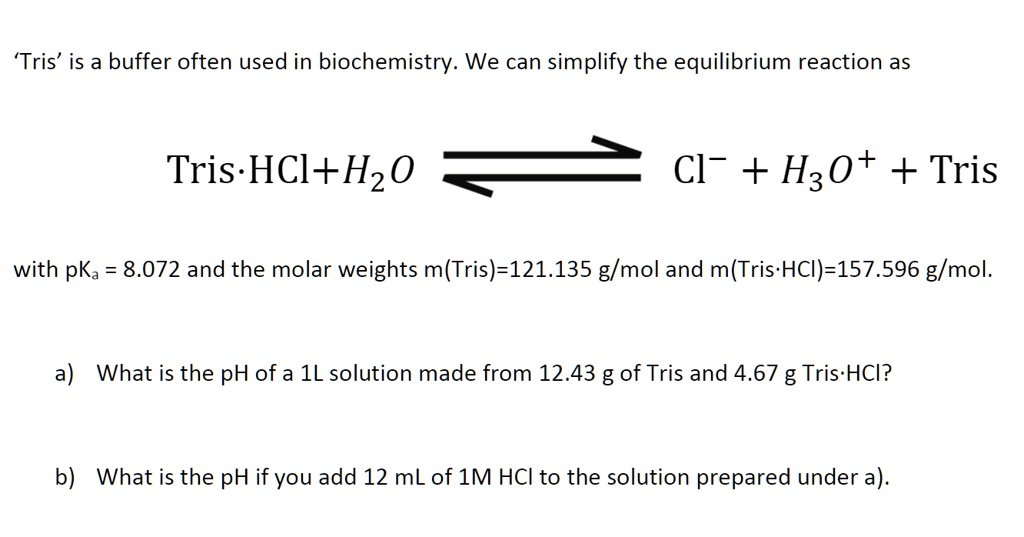
SOLVED: Tris' is a buffer often used in biochemistry. We can simplify the equilibrium reaction as: Tris-HCl + H2O -> Cl- + H3O+ + Tris with pKa = 8.072 and the molar

Interaction of Tris with DNA molecules and carboxylic groups on self-assembled monolayers of alkanethiols measured with surface plasmon resonance - ScienceDirect















![BT019b] 1M Tris-HCl, pH 8.0 | Biosolution BT019b] 1M Tris-HCl, pH 8.0 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/05/BT016-1M-Tris-HCl.jpg)

